To put it briefly, atoms are a theory that everything in the world is made of tiny (very very small) particles called atoms. This theory allows for not only understanding the basic atoms which make up everything, the air we breathe, and the food we eat.
Yet, we once had no idea these particles called atoms even existed. Scientists have been slowly making discoveries that fine-tune the model of atomic theory.
Atomic Theory Timeline: The period when we were introduced to different important milestones and so far the discoveries related to atoms.
What Is an Atom?
IntroductionAt a fundamental level, atoms are the smallest part of everything. They are so small that you cannot view them under the naked eye but they are omnipresent.
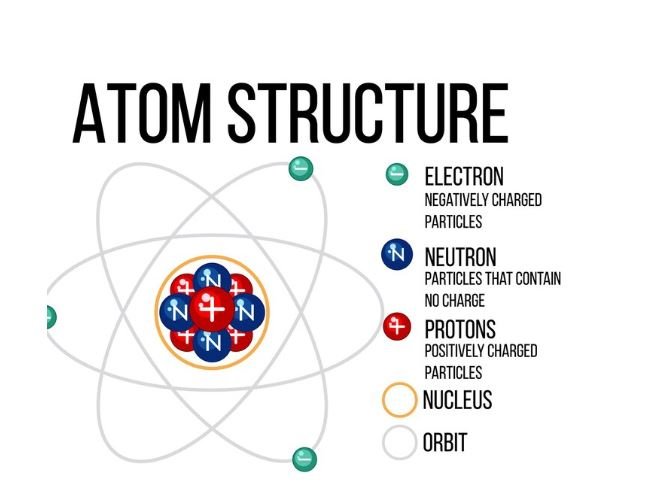
There are 3 primary components in an atom, protons, neutrons and electrons. In the middle of the atom is a clump called the nucleus, containing protons and neutrons, surrounded by electrons.
Atoms are the smallest form of matter, they are tiny building blocks of everything we see and touch. Atoms of different kinds can react together resulting in the same or new substance. Hydrogen and oxygen atoms for example combine to create water, and carbon and oxygen atoms together make carbon dioxide.
Early Ideas About Atoms
The concept of atoms is rooted as far back as ancient Greece. The first person to put serious thought into the idea of atoms was a philosopher named Democritus.
He thought that if you sliced a thing into pieces over and over, eventually there would be a piece so small it could not be cut again. He named these smallest pieces of all matter “atomos,” which in Greek meant indissoluble and is now often translated as indivisible.
Democritus had no way of looking at atoms, so his thoughts were pure conjecture. Imagination | Ideas His ideas were the start of atomic theory, scientists took a long time to learn more about atoms and prove that they exist in nature.
John Dalton’s Atomic Theory
It was not until the 19th century, that atoms came back into fashion, with a scientist by the name of John Dalton. Dalton thought that everything was formed by particles, there are atoms ( the smallest part we can divide).
He also believed that atoms are identical for the same element, but different from other elements. To give an example, he believed that since all oxygen atoms are identical and all hydrogen atoms are also the same, it made sense to think of water as being homonuclear ions.
Dalton however did not come up with ideas for no reason and his experiments allowed him to be the first scientist who could explain such things as chemical reactions and why different materials have all the properties that they do. His work it’s a leap forward in how we think of atoms and that they are real, not just some esoteric concept.
The Discovery of the Electron by J.J. Thomson
Atoms, those little balls that matter is made of have always been seen as solid building blocks until 1897 when British scientist J.J. Thomson created a model of the atom that shattered this vehicle for physics conception-dismissible little balls.
Instead, he discovered that atoms have smaller parts which are called electrons. Element → Electrons are electrons that stick to the atom just like that.
Thomson revealed that atoms had been placed inside of them, a concept unheard of at the time. He theorized that the atom appears similar to a plum pudding or blueberry muffin, where the electrons are small bits within the atom, such as blueberries in a muffin. This was known as the “plum pudding model” and is one of the first models that proved atoms are made up of different components.
How an Nucleus and Ernest Rutherford
Another scientist, Ernest Rutherford altered our way of thinking about the composition of atoms in 1911. This was a result of an experiment Rutherford did shooting tiny particles at a piece of gold foil that was very thin.
Most, he anticipated would sail right through the plate but many were reflected back to him. This led him to propose that atoms contain a tiny, solid core which he named the nucleus.
The discovery of Rutherford suggested the atom consisted mostly of space with a nucleus inside. He also noted that the nucleus is positively charged.
Rutherford demonstrated that electrons do not scatter themselves among the atom as Thomson suggested; rather they orbit around this nucleus. This was a monumental stride in understanding how atoms work and what they look like.
Niels Bohr proposed that the electrons orbit the nucleus like planets go around the sun.
Niels Bohr described how electrons move through orbits Leonard an idea based on the earlier work of Ernest Rutherford (also part of the aforementioned early 20th-century announcement ), who claimed that a nucleus sits at the centre of an atom with electrons orbiting it.
According to Bohr, electrons move in definite paths, or orbits, around the nucleus similar to the planets orbiting the sun. He suggested that every orbit had a distinct energy level, and electrons could skip from one orbit to another by consuming or throwing away the energy.
Bohr’s model was crucial in offering a transparent explanation of atom-related light emissions and chemical reactions. It revealed that electrons follow paths, or perhaps rather a better way of saying is do not just zip around any old how. Although scientists know much more about atoms today, Bohr’s model can still be used to help describe how atoms function.

James Chadwick and the Neutron Discovery
This changed, nonetheless, in 1932 a nuclear researcher James Chadwick made a significant revelation about the core of the molecule. What he found was that the nucleus has protons like they knew but more than just these particles hitting it were also these things called neutrons. Neutrons do not have an electric charge, but their mass is added to the atom and they help glue the protons together in the nucleus.
The discovery by Chadwick was significant as it provided insight into the nucleus and why it remains relatively stable, even though protons tend to push each other apart (due to both having the same charge). This only completed another piece of the atomic puzzle and further lit up the picture for scientists.
The Quantum Theory and the infrared electron cloud
Scientists went on to learn more about atoms, and in doing so they realized that Bohr was only part right and later discovered his model of electrons spinning around the nucleus in fixed orbits wasn’t true at all.
Originating during the years between the 1920s and 1930s, quantum theory was developed by the scientists Erwin Schrödinger and Werner Heisenberg. This goes right back to when people argued electrons don’t go around the nucleus in little orbits, as Bohr suggested. Instead, the electrons move in areas known as “clouds.”
They indicate the regions around a nucleus where an electron is most probably at any moment; however, not its definite locatives. Electrons behave strangely due to the quantum theory and this is what contributes to many of the properties that atoms have.
Instead, this revised conception of the electron cloud took the place of Bohr’s orbits and represents how scientists understand electrons today.
The Modern Atomic Model
Today, atoms are understood much better than in the past. Modern atomic model: Quantum theory was used to develop this modern view which said that atoms have a nucleus of very tiny mass but really dense material made up of protons and neutrons with much smaller electrons revolving around each nucleus in clouds. For example, the model explains how atoms behave and why shows why atoms join with other atoms making different types of materials.
The properties of each atom rely on the number of protons, neutrons and electrons within it. Its name indicates how many protons the atom contains an atom with one proton is hydrogen, while an atom with six protons is carbon. All elements of the periodic table are composed of different ones of these particles.
The Significance of The Atomic Theory Timeline
This history of the atomic theory is so necessary because it teaches us a lesson on how scientists have developed their understanding of the universe.
Every well, each cell that came out of the billions (and millions) was discovered after the discovery by one scientist and then other scientists Democritus, Dalton, Thomson, Rutherford, and Bohr added a new piece to the Atomic theory puzzle. Kind of fun to see how science and knowledge are always moving on, right?
You need atomic theory to clarify the foundation of all matter If we can see how atoms work, then we can understand better about chemistry, biology, physics and our environment. Understanding this allows scientists to make new materials, drugs, and technology that benefit our lives.

The Moral of Atomic Theory for Us Today
Atomic theory assists scientists in numerous fields today. Chemists use it to learn about the rate of reactions and manufacturing new materials. Doctors use it to know about the working of medicines in the body. Engineers use it to build electronics such as phones and computers. It also helps scientists understand space and the universe.
Second, it reminds us to be a little sceptical and inquisitive to remember the atomic theory. There are still things we do not understand about atoms and scientists continue to study them, but what these incredible machines have shown us has allowed our species to learn more about the universe than was ever thought possible. We can learn about atoms and keep discovering the things we learn will continue to broaden our knowledge of existence.
Conclusion
The Atomic Theory Timeline tells us how. We have learned a lot about atoms in the last 2500(ish) years since Democritus first proposed that matter was composed of indivisible building blocks at least we like to think so.
Since Democritus first dreamed up tiny, indestructible particles of matter to our current intricate quantum models, everything that we have discovered has affected our vision of the world.
The research found early discoveries made in science aim to familiarize us with the fact that it is a long process of trial and error, learning from others and asking questions.
Atomic theory helps us understand the very basic unit of anything that exists in this universe. So as we grow there is only knowledge which we can use to produce, invent and replenish the earth. The history of atomic theory reminds us that despite their minuteness, atoms can also hide the greatest secrets.